Introduction: The telomere, at the heart of the aging process

What is a telomere?
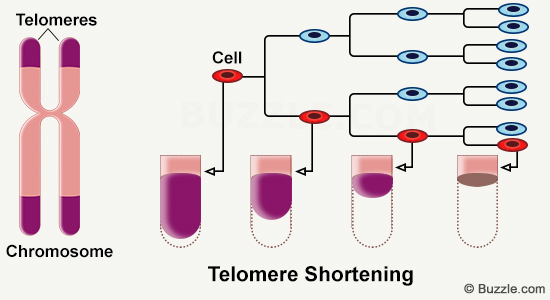
In the billions of cells that make up our body, DNA is present in the nucleus in the form of chromosomes. On these chromosomes, there are small structures, called telomeres, which gradually shorten and whose length could be linked to age.
Telomeres are ribonucleoprotein complexes found at the ends of chromosomes. They correspond to tandem repeats of nucleotide sequences (TTAGG) which decrease with each cell replication. Throughout life, cells multiply and accumulate cycles of division and replication, in order to renew damaged cells and tissues. In an aged organism, a shortening of the length of telomeres is observed [1]. Through this article, we will focus on the functioning of these telomeres and the impact of their shortening on the entire body.
Telomere function during aging
Telomere shortening is directly linked to cell division. Indeed, due to the inability of DNA polymerases to replicate the ends of linear chromosomes, a loss of genetic material is observed at each cycle of DNA replication [1]. As the telomere does not contain coding sequences, there is no loss of genomic information. Telomeres are therefore involved in the processes of preserving genome integrity and are essential for proper cellular functioning.
In the case where no mechanism comes into play to regenerate telomeres, if telomere shortening occurs with each cycle of cell replication, this indicates that the cell cannot live indefinitely. The Hayflick limit corresponds to the maximum number of cell divisions that a cell can undergo [2]. It makes it possible to make the link between the length of the telomere and the lifespan of the cell.
The telomere controls the onset of senescence, a cause of aging
The shorter the telomere, the greater the risk of losing genomic information at the next division and inducing major cellular dysfunctions. As a result, in somatic cells, when telomeres reach Hayflick's “critical” length, response pathways are activated to repair possible damage suffered by the DNA. The activation of these pathways leads to an arrest of the cell cycle, which can lead to senescence or apoptosis of the cell [3].
During the aging process, cells undergo multiple mitotic divisions and there is an increased risk of developing genetic abnormalities. Telomeres help prevent the development of these cells at the end of their life. Their measurement could thus provide information on the speed of aging and biological age.
THUS, WE QUALIFY TELOMERES AS “BILOGICAL CLOCKS” OF OUR BODY.

In addition to aging, the telomere fights against tumor cells
Tumor cells are somatic cells that have suffered dysfunctions during their development and whose multiplication is very rapid. Their proliferation leads to a large number of cell divisions and therefore accelerated shortening of telomeres. The telomeres of these cells then quickly reach the Hayflick limit and the same process described previously comes into play. An arrest of the cell cycle is induced, causing the tumor cell to enter senescence.
Telomere shortening is therefore involved in the process of stopping the proliferation of tumor cells [3]. As part of normal functioning, it helps eliminate tumors before they become malignant and turn into cancer.
Telomere and stem cells: essential during aging
However, there are mechanisms for maintaining telomere structure, which make it possible to extend the lifespan of certain cells. We find, among others, telomerase. It is the enzyme which intervenes to synthesize these telomeres [2].
This telomerase is not present in all of the body's cells. It is found active in stem cells such as HSCs, NSCs or ESCs which are respectively hematopoietic, neuronal and epidermal stem cells. These cells are mainly involved in tissue renewal processes.
The presence of telomerase in this type of cell allows them to persist over time while remaining functional. The accumulation of telomere-related disorders in this type of cells could then cause degeneration of tissues and organs, one of the main characteristics of age-associated diseases. In the fight against aging, knowledge of the biological mechanisms of telomerase seems essential.
Telomere shortening: pathologies associated with aging
Changes in telomerase lead to accelerated shortening of telomeres. This results in the appearance of diseases such as dyskeratosis congenita, a pathology which affects tissues requiring rapid and constant cell renewal, or even aplastic anemia, which causes a reduction in blood cells [2]. These diseases are associated with poor renewal of cells and tissues and present symptoms generally found during aging.
Telomere length and telomerase appear to be strongly involved in aging processes. This is why it would be interesting to include telomeres in the development of aging metrology.
SOURCES:
Katidja Allaoui on http://www.longlonglife.org/
[ 1] Chatterjee, S. (2017). Telomeres in health and disease. Journal of Oral and Maxillofacial Pathology, [online] 21(1), p.87. DOI: 10.4103/jomfp.JOMFP_39_16. [Accessed May 22, 2017].
[2] Blasco, M. (2007). Telomere length, stem cells and aging. Nature Chemical Biology, [online] 3(10), pp.640-649. DOI:10.1038/nchembio.2007.38 [Accessed 22 May 2017]
[3] Shay, J. (2016). Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discovery, [online] 6(6), pp.584-593. DOI: 10.1016/j.semcancer.2011.10.001 [Accessed 22 May 2017].


