Part 2: The influence of telomerase on telomeres and aging

The influence of telomerase on telomeres and aging
Accelerated aging due to telomerase mutations
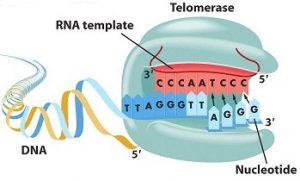
Telomerase is the enzyme responsible for synthesizing telomeres. It causes the addition of TTAGGG repeats at the chromosomal ends and allows the telomere to be reconstituted. It is made up of a protein subunit, TERT (Telomerase reverse transcriptase), responsible for telomeric synthesis as well as an RNA subunit, TERC (Telomerase RNA component), used as a synthesis model [1].
Various studies have been carried out on mice that no longer have the gene coding for the TERC subunit. The objective was to determine the impact of telomere shortening on the aging process and the onset of cancer. It was then discovered that in the absence of this gene, the long-term viability of these mice was greatly compromised [2]. Concerning the group of mice that survived, numerous symptoms associated with the loss of telomeric repeats were observed: loss of fertility, heart failure, immuno-senescence (i.e. a progressive deterioration of the immune system caused by the process of aging), a decrease in the rate of renewal of cells in the digestive system, the skin and the hematopoietic system (system of renewal of globules present in the blood)... [2]. However, all of these symptoms generally appear with age and confirm the existing link between aging and telomerase dysfunction.
Telomeres as well as telomerase appear to have an impact on aging processes. Indeed, it has been observed that mutations, affecting any component of telomerase, lead to disorders, such as dyskeratosis congenita, aplastic anemia, myelodysplastic syndromes and leukemias [1]. What these pathologies have in common is that they manifest themselves through poor cell renewal and significant tissue degeneration, signs generally associated with aging [1]. Although the exact mechanisms are not yet fully elucidated, telomerase nevertheless appears to play an important role in the aging process and lifespan.
Many pathologies associated with aging due to telomerase mutations
The study of human diseases associated with mutations in telomerase components was the starting point for the discovery of the limiting role of telomeres on longevity and the impact of telomerase on aging.
In previous articles (see: Telomeres : at the heart of aging processes ), it has been mentioned that telomeric length would be able to predict the appearance of replicative senescence, i.e. a permanent state of cycle arrest. Telomere alterations are then associated with a reduction in cellular metabolism. The accumulation of this type of cells in an organism would then lead to the manifestation of age-related phenotypes in the form of various pathologies [1].
Cardiovascular illnesses
Heart failure is one of the leading causes of premature death in older people. Researchers have therefore studied the link between the shortening of telomeres, which accelerates with age, and the development of heart disorders. They analyzed heart function in mice whose gene encoding telomerase was disabled in embryonic stem cells. In several generations of these TERC (-/-) mice, a significant decrease in life expectancy was observed due to telomere shortening, which would be coupled with a decrease in cell proliferation, an increase in apoptosis and hypertrophy (excessive increase in the volume of cardiac muscle cells) [3]. In response to these effects, ventricular dilatation, thinner vessel walls and cardiac dysfunction have been observed [3].
Thus, the acceleration in the speed of telomere shortening due to poor functioning of telomerase would be associated with diseases linked to aging such as cardiovascular diseases. In addition, other studies have demonstrated that there is a correlation between telomere shortening and premature death due to cardiovascular disease or infection [4]. Therefore, starting from the hypothesis that there would be problems with the regulation of telomerase in humans during aging, all of this information could open the way towards innovative preventive therapies against the appearance of these cardiovascular diseases.
Dementia and cognitive disorders

Researchers have demonstrated that there is a correlation between the shortening of telomeres and the appearance of mental disorders: an increase in perceived stress, the development of cognitive disorders and depressive states [5]. Additionally, in individuals experiencing accelerated telomere shortening due to telomerase dysfunction, the development of schizophrenia and mood disorders has also been observed [6]. These different brain disorders, which frequently develop with age, confirm the central role of telomerase and telomeres in the aging process.
Poor cell renewal and tissue degradation
Among the pathologies associated with TERC mutations, we find diseases leading to poor cell renewal. This is, for example, the case of dyskeratosis congenita, also called telomeropathy. Indeed, it manifests itself by a very rapid degradation of telomeres. Although the clinical signs are very numerous, the most severe effects are: bone marrow aplasia (rareness of bone marrow), neutropenia (decrease in the quantity of certain white blood cells, polynuclear neutrophils), thrombocytopenia (decrease in platelets) , pulmonary fibrosis, global immune deficiency and the occurrence of cancer. There is currently no specific treatment for this disease, other than hematopoietic stem cell transplantation, which appears to have positive effects [2].
These mutations in telomerase components contribute to the degradation of the body at all levels: poor cell renewal, disruption of the body's protection and repair systems, tissue degeneration, etc. [2]. The pathologies associated with it are numerous. We find aplastic anemia which is characterized by a too low number of red blood cells in the blood or even idiopathic pulmonary fibrosis which is a fatal disease characterized by scarification of the lungs leading to respiratory disorders.
The origin of telomerase dysfunction and accelerated aging
Although the effects of these telomerase mutations vary greatly from one individual to another, they have in common that they are associated with defects in cell renewal and tissue regeneration. They contribute to the development of phenotypes comparable to signs of aging.
Telomeres are essential for maintaining the cell cycle. However, while it has been shown that dysregulation of telomerase enzymatic activity can accelerate aging, overexpression of this enzyme could induce excessive cell proliferation and therefore potentially increase the risk of developing tumors [2]. . Therefore, regarding future therapeutic avenues, it would be interesting to move towards targeted treatments. A telomere therapy targeting only stem cells would make it possible to overcome the problem of cell renewal with age without the risk of inducing the development of cancer throughout the body.
SOURCES:
Katidja Allaoui on http://www.longlonglife.org/
Sources:
[1] Chatterjee, S. (2017). Telomeres in health and disease. Journal of oral and maxillofacial pathology: JOMFP, 21(1), 87.
[2] Blasco, M.A. (2007). Telomere length, stem cells and aging. Nature chemical biology, 3(10), 640-649.
[3] Leri, A., Franco, S., Zacheo, A., Barlucchi, L., Chimenti, S., Limana, F., … & Blasco, MA (2003). Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. The EMBO journal, 22(1), 131-139.
[4] Cawthon, RM, Smith, KR, O'Brien, E., Sivatchenko, A., & Kerber, RA (2003). Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet, 361(9355), 393-395.
[5] Canela, A., Vera, E., Klatt, P. & Blasco, MA High-thoughput telomere length quantification by FISH and its application to human population studies. Proc. Natl. Acad. Sci. USA 104, 5300–5305 (2007).
[6] Teyssier, JR, Ragot, S., Donzel, A., & Chauvet-Gelinier, JC (2010). Telomere length in the cortex of patients with depressive disorders. L’Encephale, 36(6), 491-494.
*https://planet-vie.ens.fr/article/1813/invalidation-gene-knock-out


